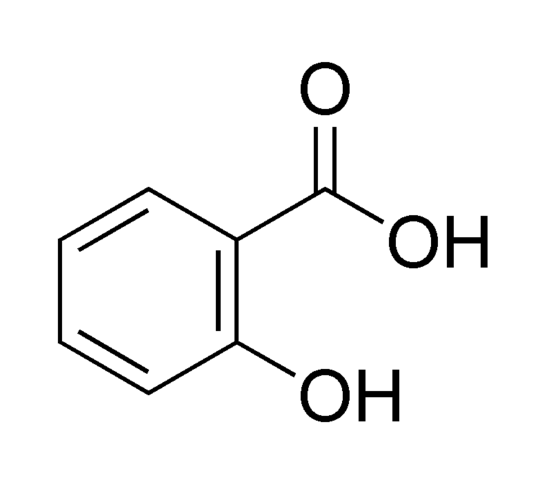
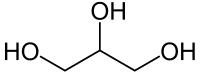
In a small amount necessary for life
Not a source of energy for the body
| Source: http://www.nactalia.com/ |
Water-soluble vitamins
Vitamin C
It helps absorbing iron, forming collagen and red blood cells
Deficiency causes bleeding, gingivitis
Source: Vegetables - peppers, cabbage
Fruit - strawberries, red currants, oranges, lemons
Vitamin B1
Involved in carbohydrate metabolism
Deficiency causes cramps, fatigue, digestive disorders
Source: cereals, yeast, legumes, offal
Vitamin B2
Deficiency causes inflammation of the corners of the mouth, skin lesions and mucous membranes
Source: meat, milk, eggs, liver
| Source: http://alternativa-za-vas.com/ |
Supporting growth and cell division
Deficiency causes skin diseases, fatigue
Source: eggs, liver, meat, yeast
Fat-soluble vitamins
Vitamin A
Component of the visual pigment
Deficiency causes night blindness, growth arrest
Source: liver, egg, meat, cheese, fat seafood
Vitamins D
Group of vitamins - most important is D2 and D3
Promotes the metabolism of calcium and phosphorus
Deficiency causes softening and deformation of bones
Source: meat, liver, UV-fat seafood
| Source: http://www.calivita.si/ |
Vitamin E
Protects cell membranes, supports the activity of the gonads
Deficiency causes muscle weakness, impaired vascular system
Source: vegetable oils
Vitamin K
It participates in the blood coagulation process
Deficiency causes blood clotting disorder
Source: leafy vegetables, formed by intestinal bacteria